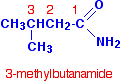|
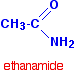
INTRODUCING AMIDES This page explains what amides are and looks at their simple physical properties such as solubility and melting points. What are amides? Amides are derived from carboxylic acids. A carboxylic acid contains the -COOH group, and in an amide the -OH part of that group is replaced by an -NH2 group. So . . . amides contain the -CONH2 group. Some simple amides The most commonly discussed amide is ethanamide, CH3CONH2 (old name: acetamide).
The three simplest amides are:
Notice that in each case, the name is derived from the acid by replacing the "oic acid" ending by "amide". If the chain was branched, the carbon in the -CONH2 group counts as the number 1 carbon atom. For example:
Physical properties Melting points Methanamide is a liquid at room temperature (melting point: 3°C), but the other amides are solid. For example, ethanamide forms colourless deliquescent crystals with a melting point of 82°C. A deliquescent substance is one which picks up water from the atmosphere and dissolves in it. Ethanamide crystals nearly always look wet. | |||||||
|
Note: Ethanamide is said to smell of mice. In fact, the smell is due to an impurity in the ethanamide called N-methylethanamide, CH3CONHCH3, where one of the hydrogens in the -NH2 group has been replaced by a methyl group. | |||||||
|
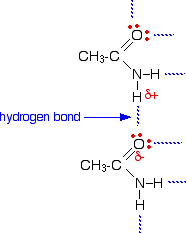
The melting points of the amides are high for the size of the molecules because they can form hydrogen bonds. The hydrogen atoms in the -NH2 group are sufficiently positive to form a hydrogen bond with a lone pair on the oxygen atom of another molecule.
As you can see, there is the potential for lots of hydrogen bonds to be formed. Each molecule has two slightly positive hydrogen atoms and two lone pairs on the oxygen atom. These hydrogen bonds need a reasonable amount of energy to break, and so the melting points of the amides are quite high. | |||||||
|
Note: If you aren't happy about hydrogen bonding then you really ought to follow this link before you go on. Use the BACK button on your browser to return to this page. | |||||||
|
Solubility in water The small amides are soluble in water because they have the ability to hydrogen bond with the water molecules. It needs energy to break the hydrogen bonds between amide molecules and between water molecules before they can mix - but enough energy is released again when the new hydrogen bonds are set up to allow this to happen.
© Jim Clark 2004 (modified February 2016) |
|||||||