|
EXPLAINING THE NUCLEOPHILIC SUBSTITUTION REACTIONS BETWEEN HALOGENOALKANES AND HYDROXIDE IONS
This page guides you through the nucleophilic substitution mechanisms for the reactions between halogenoalkanes and hydroxide ions from, for example, sodium hydroxide. | |
|
Important! It would help if you first read the page What is nucleophilic substitution? before you go on. You must also be clear about the differences between primary, secondary and tertiary halogenoalkanes. | |
|
The reactions between primary or secondary halogenoalkanes and hydroxide ions - the SN2 mechanism
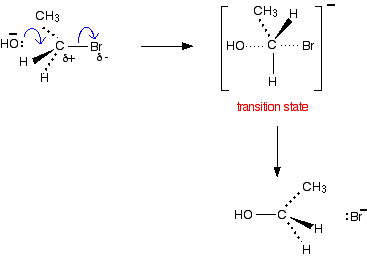
Hydroxide ions as nucleophiles A nucleophile is a species (an ion or a molecule) which is strongly attracted to a region of positive charge in something else. Nucleophiles are either fully negative ions, or else have a strongly In the case of the hydroxide ion, there is a full negative charge on the oxygen, as well as three lone pairs of electrons. The nucleophilic substitution reaction - an SN2 reaction We'll talk this reaction through with a primary halogenoalkane to start with, taking bromoethane as typical. The bromoethane has a polar bond between the carbon and the bromine. | |
|
Note: In an exam you must show the lone pair of electrons on the nucleophile (in this case, the OH- ion). It probably doesn't matter whether you show them on the departing Br- ion or not. | |
|
One of the lone pairs on the OH- ion will be strongly attracted to the At some point during this, the -OH group and the bromine will both be half-attached to the carbon. This is called the transition state for the reaction. It isn't an intermediate - you can't isolate it and it doesn't have any independent existence. It's just the half-way stage of a smooth movement of atoms and electrons. The movement goes on until the -OH is firmly attached to the carbon, and the bromine has been expelled as a Br- ion. You may need to show the formation of the intermediate in the mechanism (depending on what your examiners want). It simply needs you to draw the mechanism showing some more detail about how the various groups are arranged in space.
Be very careful when you draw the transition state to make a clear difference between the dotted lines showing the half-made and half-broken bonds, and those showing the bonds going back into the paper. Notice that the molecule has been inverted during the reaction - rather like an umbrella being blown inside-out. | |
|
Note: If you aren't happy about the various ways of drawing bonds, it is important to follow this link to find out exactly what the various symbols mean. It is also important to know which of these ways of drawing the mechanism your particular examiners want you to use. If you haven't already checked your syllabus, recent exam papers and mark schemes, you must do so! At the time of writing, Edexcel, for example, wanted the transition state included, and that isn't obvious from their syllabus. You have to check mark schemes and examiners reports. Use the BACK button on your browser to return to this page. | |
|
Technically, this is known as an SN2 reaction. S stands for substitution, N for nucleophilic, and the 2 is because the initial stage of the reaction involves two species - the bromoethane and the OH- ion. If your syllabus doesn't refer to SN2 reactions by name, you can just call it nucleophilic substitution. The SN2 reaction in secondary halogenoalkanes The reaction can happen in exactly the same way with a secondary halogenoalkane, although they also have the potential for reacting via a different mechanism (which we'll deal with shortly). Again, a lone pair on the approaching hydroxide ion forms a bond with the
The reactions between secondary or tertiary halogenoalkanes and hydroxide ions - the SN1 mechanism | |
|
Note: Are you sure your syllabus wants this? Several syllabuses restrict you to primary halogenoalkanes. Haven't got a syllabus? If you are working to a UK-based syllabus for 16 - 18 year olds, follow this link to find out how to get one. | |
|
To start with, we'll talk this mechanism through with a simple tertiary halogenoalkane like the one on the right (2-bromo-2-methylpropane).
Why do tertiary halogenoalkanes need a different mechanism? When a nucleophile attacks a primary halogenoalkane, it approaches the With a tertiary halogenoalkane, this approach from the back is impossible. The back of the molecule is completely cluttered with CH3 groups. The SN1 mechanism The reaction happens in two stages. In the first, a small proportion of the halogenoalkane ionises to give a carbocation (carbonium ion) and a bromide ion. This reaction is possible because tertiary carbocations are relatively stable compared with secondary or primary ones. Even so, the reaction is slow. | |
|
Note: Not sure about the stability of carbocations (carbonium ions)? Follow this link if you need to find out. | |
|
Once the carbocation is formed, however, it would react immediately it came into contact with an OH- ion. The lone pair on the nucleophile is strongly attracted towards the positive carbon, and moves towards it to create a new bond. How fast the reaction happens is going to be governed by how fast the halogenoalkane ionises - because that's a slow process. Because this initial slow step only involves one species, the mechanism is described as SN1 - substitution, nucleophilic, one species taking part in the initial slow step. The SN1 mechanism in secondary halogenoalkanes Secondary halogenoalkanes (like 2-bromopropane) can use either the SN1 or the SN2 mechanism. The back of the molecule is rather more cluttered than in a primary halogenoalkane, but there is still room for the lone pair on the nucleophile to approach and form a bond. We've already dealt with that reaction. It is also possible to get some slight ionisation of the halogenoalkane to give an SN1 mechanism, but this reaction is much less successful than with tertiary halogenoalkanes, because the secondary carbocation formed isn't as stable as a tertiary one. Once the carbocation has been formed, it will react immediately with a hydroxide ion. A lone pair on the hydroxide ion is strongly attracted to the positive carbon, moves towards it, and forms a bond.
To menu of nucleophilic substitution reactions. . . © Jim Clark 2000 (modified 2004) |
|