|
ORBITS AND ORBITALS This page tries to explain why the idea that electrons orbit a nucleus like planets around the sun is wrong. A worrying number of students believe in this picture and it causes them lots of problems. If you have come to this page straight from a search engine, you might find it more useful to read the main page about atomic orbitals first and follow the link on that page back to here later if you need to. Most popular pictures of the atom are wrong! Most popular science pictures of the atom show electrons moving around a nucleus like planets around the sun. These pictures are quite simply wrong. They come from an old idea about the structure of the atom and have lasted, partly from habit, and partly because the modern view of the arrangement of electrons in the atom is too difficult to draw simple pictures of.
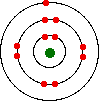
You might, of course, say "Well, I know it isn't like this. The electrons are arranged in a particular pattern - for example, in a diagram like this one for sodium:"
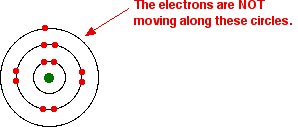
That's fine - as long as you understand what the circles mean. The circles are NOT orbits. The electrons are NOT moving around the nucleus along the circles.
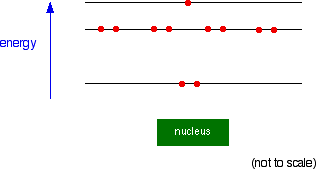
Instead, the circles represent energy levels. The electrons on the circle closest to the nucleus have the lowest energy. The eight electrons on the next circle have a higher energy, and the one on the outer circle has the highest energy. You can quite usefully straighten the circles out and draw the sodium as in the next diagram. By doing this, any sense of movement around the nucleus disappears.
| |
|
Note: This diagram is slightly simplified. As you will find when you learn more about the arrangement of electrons, the eight electrons in the second energy level don't all have exactly the same energy. Two of them have a slightly lower energy than the other six. It doesn't affect the current discussion in any way. | |
|
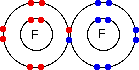
So . . . how are the electrons moving around the nucleus? The short answer to this is that we don't know. In fact, it's more than that - current theory says that it is impossible to know. If you have planets in orbit around the sun, it is possible to predict exactly how they are going to move in future. In other words it is possible to plot out an orbit for them in mathematical terms so that you can know exactly where they are going to be next week, next year or in 100 years time. You can't do that for electrons. To plot an orbit, you need to know, amongst other things, exactly where the electron is, what direction it is heading in, and how fast it is going. If you have come to this page from the Atomic Orbitals page, you will have read about the Heisenberg Uncertainty Principle which says, loosely, that you can't know with certainty both where an electron is and where it is going next. (You will find a better definition on the Orbitals page.) If we can't know these essential facts, then we have no idea what the electrons are actually doing in the atom. All we can know about them is their energy and where we are most likely to find them. Any particular electron will be found in a region of space known as an orbital. Orbitals come in all sorts of different shapes and sizes, and you can read about some of these on the Orbitals page. An orbital is just a bit of space where there is a 95% chance of finding that particular electron. If an electron is in a particular orbital, you know about its energy - but there is no way of knowing how it is moving around within that orbital. The big problem in all of this, to my mind, is the confusion in the words "orbit" and "orbital". They sound as if they refer to something very similar. They don't! It would have been much better if orbitals had been called something entirely different. The question of covalent bonding This is the main area where I get questions that suggest to me that the student has got totally confused about the difference between an orbit and an orbital. The question tends to be about a simple covalent bond, for example in a fluorine molecule, which is often drawn as:
The question then says something like "Does the bonding pair of electrons move around both of the nuclei, or does it stop between the two atoms?" If you try to think of the circles as orbits, then it is reasonable to picture the bonding pair of electrons doing a sort of figure-of-eight looping around both nuclei. But the circles AREN'T orbits, and so this picture is completely false. What happens when a covalent bond is formed between two fluorine atoms is that an orbital from one atom overlaps in space with one from another atom. Both of these originally contained one electron. They combine to make a new orbital containing both electrons- a molecular orbital. | |
|
Note: In fact, two atomic orbitals combine to make two new molecular orbitals. One is called a bonding orbital (which is what we normally talk about at this level), the other an anti-bonding orbital (which is normally empty). Don't worry about that for now - or possibly not ever! | |
|
This orbital will be a different shape from the original ones. We have no idea how those electrons are moving around within that new orbital. You will come across lots more about this when you look at the bonding in compounds like methane, ethane, ethene or benzene. Why do so many people find this so confusing? The answer lies in the careless and thoughtless way this is often taught at the point where you first meet atomic structure - either in a classroom or in some textbooks. The sequence might go like this:
And all this is so easily avoided! The sequence should go something like this:
Return to the page about atomic orbitals . . . © Jim Clark 2006 |
|