|
THE CONTACT PROCESS This page describes the Contact Process for the manufacture of sulphuric acid, and then goes on to explain the reasons for the conditions used in the process. It looks at the effect of proportions, temperature, pressure and catalyst on the composition of the equilibrium mixture, the rate of the reaction and the economics of the process. | ||
|
Important: If you aren't sure about using Le Chatelier's Principle or about the effect of changing conditions on rates of reaction you should explore these links before you go on. When you are reading this page, if you find that you aren't understanding the effect of changing one of the conditions on the position of equilibrium or on the rate of the reaction, come back and follow up these links. | ||
|
A brief summary of the Contact Process The Contact Process:
Making the sulphur dioxide This can either be made by burning sulphur in an excess of air:
. . . or by heating sulphide ores like pyrite in an excess of air:
In either case, an excess of air is used so that the sulphur dioxide produced is already mixed with oxygen for the next stage. Converting the sulphur dioxide into sulphur trioxide This is a reversible reaction, and the formation of the sulphur trioxide is exothermic.
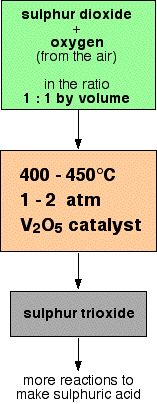
A flow scheme for this part of the process looks like this:
The reasons for all these conditions will be explored in detail further down the page. Converting the sulphur trioxide into sulphuric acid This can't be done by simply adding water to the sulphur trioxide - the reaction is so uncontrollable that it creates a fog of sulphuric acid. Instead, the sulphur trioxide is first dissolved in concentrated sulphuric acid:
The product is known as fuming sulphuric acid or oleum. This can then be reacted safely with water to produce concentrated sulphuric acid - twice as much as you originally used to make the fuming sulphuric acid.
Explaining the conditions The proportions of sulphur dioxide and oxygen The mixture of sulphur dioxide and oxygen going into the reactor is in equal proportions by volume. Avogadro's Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. That means that the gases are going into the reactor in the ratio of 1 molecule of sulphur dioxide to 1 of oxygen. That is an excess of oxygen relative to the proportions demanded by the equation.
According to Le Chatelier's Principle, Increasing the concentration of oxygen in the mixture causes the position of equilibrium to shift towards the right. Since the oxygen comes from the air, this is a very cheap way of increasing the conversion of sulphur dioxide into sulphur trioxide. Why not use an even higher proportion of oxygen? This is easy to see if you take an extreme case. Suppose you have a million molecules of oxygen to every molecule of sulphur dioxide. The equilibrium is going to be tipped very strongly towards sulphur trioxide - virtually every molecule of sulphur dioxide will be converted into sulphur trioxide. Great! But you aren't going to produce much sulphur trioxide every day. The vast majority of what you are passing over the catalyst is oxygen which has nothing to react with. By increasing the proportion of oxygen you can increase the percentage of the sulphur dioxide converted, but at the same time decrease the total amount of sulphur trioxide made each day. The 1 : 1 mixture turns out to give you the best possible overall yield of sulphur trioxide. The temperature Equilibrium considerations You need to shift the position of the equilibrium as far as possible to the right in order to produce the maximum possible amount of sulphur trioxide in the equilibrium mixture. The forward reaction (the production of sulphur trioxide) is exothermic.
According to Le Chatelier's Principle, this will be favoured if you lower the temperature. The system will respond by moving the position of equilibrium to counteract this - in other words by producing more heat. In order to get as much sulphur trioxide as possible in the equilibrium mixture, you need as low a temperature as possible. However, 400 - 450°C isn't a low temperature! Rate considerations The lower the temperature you use, the slower the reaction becomes. A manufacturer is trying to produce as much sulphur trioxide as possible per day. It makes no sense to try to achieve an equilibrium mixture which contains a very high proportion of sulphur trioxide if it takes several years for the reaction to reach that equilibrium. You need the gases to reach equilibrium within the very short time that they will be in contact with the catalyst in the reactor. The compromise 400 - 450°C is a compromise temperature producing a fairly high proportion of sulphur trioxide in the equilibrium mixture, but in a very short time. The pressure Equilibrium considerations
Notice that there are 3 molecules on the left-hand side of the equation, but only 2 on the right. According to Le Chatelier's Principle, if you increase the pressure the system will respond by favouring the reaction which produces fewer molecules. That will cause the pressure to fall again. In order to get as much sulphur trioxide as possible in the equilibrium mixture, you need as high a pressure as possible. High pressures also increase the rate of the reaction. However, the reaction is done at pressures close to atmospheric pressure! Economic considerations Even at these relatively low pressures, there is a 99.5% conversion of sulphur dioxide into sulphur trioxide. The very small improvement that you could achieve by increasing the pressure isn't worth the expense of producing those high pressures. The catalyst Equilibrium considerations The catalyst has no effect whatsoever on the position of the equilibrium. Adding a catalyst doesn't produce any greater percentage of sulphur trioxide in the equilibrium mixture. Its only function is to speed up the reaction. Rate considerations In the absence of a catalyst the reaction is so slow that virtually no reaction happens in any sensible time. The catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reactor. | ||
|
Note: If you are interested in the mechanism for the catalytic reaction you will find it on an introductory page on types of catalysts. Use the BACK button on your browser if you want to return to this page. | ||
© Jim Clark 2002 (modified April 2013) |
||