|
THE EFFECT OF CATALYSTS ON REACTION RATES This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates, and with the Maxwell-Boltzmann distribution of molecular energies in a gas. | |||||||||||||
|
Note: If you haven't already read the page about collision theory, you should do so before you go on. Use the BACK button on your browser to return to this page, or come back via the rates of reaction menu. | |||||||||||||
|
Note that this is only a preliminary look at catalysis as far as it affects rates of reaction. If you are looking for more detail, there is a separate section dealing with catalysts which you can access via a link at the bottom of the page. The facts What are catalysts? A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of catalyst as you had at the beginning. Some examples Some common examples which you may need for other parts of your syllabus include:
| |||||||||||||
|
Note: You can find details of these and other catalytic reactions by exploring the menu for the main section on catalysis. You will find a link at the bottom of this page. | |||||||||||||
|
The explanation The key importance of activation energy Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. | |||||||||||||
|
Note: What follows assumes you have a reasonable idea about activation energy and its relationship with the Maxwell-Boltzmann distribution. This is covered on the introductory page about collision theory. If you aren't confident about this, follow this link, and use the BACK button on your browser to return to this page. | |||||||||||||
|
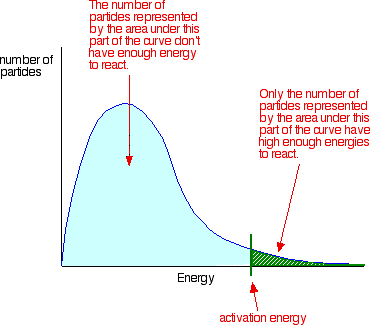
You can mark the position of activation energy on a Maxwell-Boltzmann distribution to get a diagram like this:
Only those particles represented by the area to the right of the activation energy will react when they collide. The great majority don't have enough energy, and will simply bounce apart. If there are very few particles with enough energy at any time, then the reaction will be slow. | |||||||||||||
|
Important: I have already commented on this on the introductory page about collision theory (and also on the page about the effect of temperature). You mustn't get the idea that those particles in the blue area of the graph can never react. There are constant random collisions between the particles, and constant exchanges of energy between them. Some particles will gain energy in random collisions, and others will lose energy. So a low energy particle could, an instant later, have gained enough energy from a collision that it could now react. And the opposite is true. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. Because of this constant exchange of energy, given time all the particles will react if the reacting proportions are right. | |||||||||||||
|
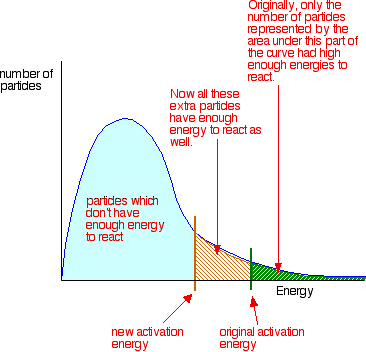
Catalysts and activation energy To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. In other words, to move the activation energy on the graph like this:
As before, particles which don't have enough energy at a particular time will at some time in the future gain energy from random collisions, just as other particles will lose energy. You mustn't get the idea that those particles in the blue area of the graph can never react - given time they will.
Adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides an alternative route for the reaction. That alternative route has a lower activation energy. Showing this on an energy profile:
A word of caution!
It does not "lower the activation energy of the reaction". There is a subtle difference between the two statements that is easily illustrated with a simple analogy. Suppose you have a mountain between two valleys so that the only way for people to get from one valley to the other is over the mountain. Only the most active people will manage to get from one valley to the other. Now suppose a tunnel is cut through the mountain. Many more people will now manage to get from one valley to the other by this easier route. You could say that the tunnel route has a lower activation energy than going over the mountain. But you haven't lowered the mountain! The tunnel has provided an alternative route but hasn't lowered the original one. The original mountain is still there, and some people will still choose to climb it. In the chemistry case, if particles collide with enough energy they can still react in exactly the same way as if the catalyst wasn't there. It is simply that the majority of particles will react via the easier catalysed route.
To the more detailed catalysis menu . . . To the rates of reaction menu . . . © Jim Clark 2002 (last modified October 2018) |
|||||||||||||