|
QUESTIONS AND COMMENTS What exactly does the antiseptic TCP contain? The problem If you live in the UK, you are almost bound to have come across the mild antiseptic TCP. Throughout the whole of my teaching career, I had always assumed that the initials stood for the molecule 2,4,6-trichlorophenol:
However, someone e-mailed me to say that they had found a reference on Wikipedia (up to August 2007 - now modified) which implied that the main ingredient in TCP was trichlorophenylmethyliodosalicyl. As a result (and because I wasn't entirely convinced), I removed all references to TCP from Chemguide apart from a temporary note at the bottom of the Main Menu pointing this out. About a month later, I had another e-mail from someone who thought that TCP was indeed 2,4,6-trichlorophenol - and quoted a number of organic textbooks which said so. At that point, it seemed to me that the only sensible thing to do was to contact the manufacturers and ask them. The readily available web sources all said that TCP was manufactured by Pfizer. That turns out not to be true any more. The UK firm Chefaro (a subsidiary of Omega Pharma) acquired the brand in 2004. The reliable information I now have comes from their brand manager. The facts as far as I know them She tells me that the antiseptic TCP originally contained trichlorophenylmethyliodosalicyl, but that it was replaced by other active ingredients in the 1950s. (Update September 2015: That date may well be a bit late. See the photo below.) In other words, although the name TCP derives from trichlorophenylmethyliodosalicyl, TCP no longer contains it - and hasn't contained it for at least 60 or 70 years! At the time I am writing this (prior to the correction on Wikipedia), Wikipedia and all the other sites which relied on it were giving wrong information. TCP liquid now contains a dilute solution of phenol and halogenated phenols. The active ingredients are:
Other ingredients are: Glycerol, Concentrated Phosphoric Acid, E104 (Quinoline Yellow), Water | |||||
|
Note: These are the latest (2022) figures. The figures come from information that you can find on current (2022) bottles of TCP liquid. As you will see later, the detailed composition has changed over time. In the table, "0.68% w/v", for example, means that if you had 100 cm3 of solution, it would contain 0.68 g of solute. | |||||
|
Notice that the table talks about "halogenated" phenols. I haven't been able to find any reliable information about which halogens are used in the modern antiseptic, and how many of them are substituted into the phenol ring. The name suggests a mixture. Whether that mixture contains any 2,4,6-trichlorophenol, I have no idea. Neither do I have any idea about the identity of the compound that TCP is named after (trichlorophenylmethyliodosalicyl). There is nowhere on the web that I have been able to find that gives a structure for it. The problem, of course, is that although it looks superficially like a modern IUPAC name, it isn't. TCP was first produced in 1918 - and naming that far back was pretty haphazard. My contact at Chefaro tells me that their archives contain no records of the nature of the molecule. | |||||
|
Note: If any one reading this has a reliable structure for trichlorophenylmethyliodosalicyl, please contact me via the address on the about this site page. In February 2013 I had an interesting e-mail from Steve Hunter suggesting that the name trichlorophenylmethyliodosalicyl might actually not be real. You can find a copy of this email (with thanks to Steve) by following this link. This is just speculation of course, but this TCP page has been online since 2007 and nobody has so far suggested to me what its structure might be. Another email - this time from Antoni Lachetta in March 2020: He made the interesting suggestion that the word trichlorophenylmethyliodosalicyl could be derived from a combination of two names, trichlorophenol and an iodinated version of methyl salicylate (oil of wintergreen), perhaps with one or more iodine atoms substituted into the benzene ring. Methyl salicylate is apparently still used as one of the ingredients in Listerene, an antiseptic mouthwash. Who knows! | |||||
|
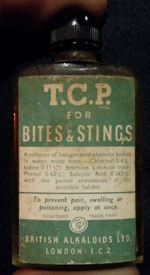
Update September 2015: Tom Dodds sent me a photo of an old bottle of TCP dating probably somewhere between the mid-1930s and mid-1940s, for which I am very grateful. This goes some way to listing what went in to making the TCP, although not the actual compounds.
The ingredients list is too small to read on this web version, but it says: A solution of halogenated phenolic bodies in water, made from :- Chlorine 0.4%; Iodine 0.11%; Bromine a minute trace; Phenol 0.63%; Salicylic acid 0.045%; with the partial elimination of the ionisable halides . Update June 2022: Pekka Luoma sent me a photo of a box of TCP ointment with an expiry date of October 1999, but which also included the composition of TCP liquid at that time. It then contained sodium salicylate in addition to the phenol and halogenated phenols which were in exactly the same amounts as in the table above. So the exact composition of TCP liquid changes with time. The ointment, by the way, had a lot of additional ingredients including methyl salicylate and salicylic acid. Adding to the confusion! TCP is a brand name for a mild antiseptic, originally based on the word trichlorophenylmethyliodosalicyl, but no longer containing it. Unfortunately TCP is also a commonly used abbreviation for trichlorophenol (which may or may not be one of the components of the "halogenated phenols" present in a bottle of TCP antiseptic!). 2,4,6-trichlorophenol (together with a whole lot of other trichlorophenols where the three chlorines are arranged differently around the benzene ring) is used as a pesticide, bactericide and fungicide. In other words, it is pretty good at killing things! It is also a suspected carcinogen. The fact that it is fairly unpleasant doesn't, of course, mean that it couldn't be used safely in very dilute solution, and so doesn't exclude the possibility that it may be there in small quantities in TCP antiseptic. (See below.) Update July 2014 I have had an e-mail from a specialist in Pharmaceutics at Aston University who makes a number of useful points. Perhaps the most important is that TCP is likely to contain different active ingredients depending on whether you buy it as an ointment or a cream or a liquid. Halogenated phenols aren't very water soluble, and so if your TCP is sold as an aqueous cream or a liquid, it may well contain different antimicrobial compounds than an oil-based ointment. On the toxicity problem, he points out that halogenated phenols aren't absorbed by the skin very well, so using them externally shouldn't cause a problem. And finally he notes that, certainly in early versions of TCP, there was almost certainly a mixture of products from the halogenation process, containing all possible substitutions of halogen atoms into the phenol ring. I suspect that may be why the current formulation of TCP liquid mentioned above contains "halogenated phenols" rather than specifically named ones. Update September 2021 I have had a couple of e-mails from someone who actually worked with the production process of TCP. I will let the e-mails speak for themselves. First e-mail
Second e-mail
I also had a follow-up e-mail to those two which included the interesting sentence:
These days salicylic acid is used in acne remedies. It apparently works by helping the skin to shed dead cells and by decreasing redness and swelling. Whether or not that was the reason for using it, I don't know. So, my guess is that the original name was just a tacked together list of all the bits that went into the production process. That just leaves "methyl" unaccounted for. Shortly after all this I had an email from a teacher who wondered whether what was added in the process was actually methyl salicylate rather than salicylic acid. That might account for the "methyl". Methyl salicylate is commonly known as "oil of wintergreen", and is used in various things you rub on your skin to relieve pain, so it might be logical to add it to TCP. So I contacted my correspondent from above, who said what was added was . . .
That must have been salicylic acid. Methyl salicylate is a liquid - its melting point is -9°C. It certainly isn't a crystalline solid which might be supplied in sacks! So that speculation, attractive as it was, bites the dust. To be honest, I think this has gone as far as it can. TCP formulations have changed with time, and who knows what it contained when it was first produced prior to the information on this page. So, please, unless you have any definite evidence rather than speculation, no more emails! Life is too short. The moral to all this The really important point I want to make isn't about what TCP contains or doesn't contain. It is about the use of information you find on the internet. It is very easy to assume that because you find some bit of information all over the internet it must be right. It doesn't work like that! I found lots of references to TCP antiseptic containing trichlorophenylmethyliodosalicyl, despite the fact that it hasn't had this substance in it for at least 60 or 70 years (even if it ever was a real substance rather than a made-up name), but many of these references could be tracked back to the same Wikipedia article. In other cases, the wording was sufficiently similar that although the source wasn't acknowledged, it probably came from Wikipedia as well. The internet is a potentially dangerous tool. One single bit of misinformation can get multiplied over huge numbers of web sites until a Google search for it will produce page after page all pointing at the same false information on different sites - and the truth (assuming anybody knows it) gets completely swamped out. And there is no control over this - for the vast majority of web sites, there is no editor checking your facts, and there are no reviewers criticising every sloppy statement. Because you find a bit of information on the internet, you can't assume that it is true. Remember that every time you do an internet search! | |||||
|
Note: Following the logic of this, why should you believe that what you find on Chemguide is true? I have to say to start with that lots of it may not be literally "true". All I would claim is that it represents the closest we can get to the truth given the state of current knowledge and understanding - and allowing for the simplifications necessary to make it understandable to my target audience. The best guarantee of accuracy lies in the hundreds of thousands of people who read Chemguide every month - including some very high powered people. If people find things they disagree with, they write and tell me, and it gets corrected. Fortunately this doesn't happen very often, but this particular page is a good example of how the process of trying to get things right works. I spend ridiculous amounts of time trying to get things right to start with, but I am only human and mistakes happen. In those cases, I am happy to accept criticism and make the necessary changes. | |||||
|
Return to questions list . . . © Jim Clark 2007 (last updated June 2022) |
|||||